Retail’s battle with ‘dupe’ culture — how brands like Lululemon are fighting back

We explore recent examples of how brands are responding to dupe culture and outline practical steps that retail businesses can take to protect their brand.
Read more
We make the difference. Talk to us: 0333 004 4488 | hello@brabners.com
AuthorsNatalie Hardman
6 min read
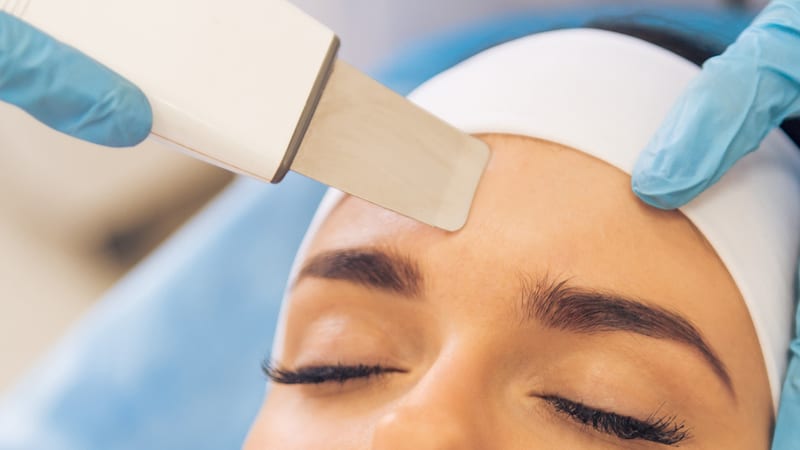
The much-anticipated Government consultation for the licensing of non-surgical cosmetic procedures (the Consultation) finally opened on 2 September 2023.
It’s now the final call for responses to the Consultation from industry professionals and consumers who have undergone procedures, which must be filed online by 11:59 on 28 October 2023.
The feedback received in response to the Consultation will help to shape the new licensing regime and the regulations to be put in place.
Here, Associate Natalie Hardman and Trainee Solicitor Mpho Kgatuke outline the purpose of the consultation and why feedback is so critical to shaping the future of the non-surgical cosmetic procedures industry.
We previously reported on the rise and impact of non-surgical cosmetic procedures in the beauty industry and that it was clear that there is a lack of substantial regulation in this area and for those who can perform non-surgical cosmetic procedures.
In April 2022, the Health and Care Act was amended to give the Secretary of State for Health and Social Care the power to introduce a licensing scheme to regulate non-surgical cosmetic procedures in England.
Public safety within the industry is a key concern and there are few safety nets currently in place to regulate the profession and protect the public. Ultimately, by adopting a licensing regime for non-surgical cosmetic procedures, a consistent industry standard will be provided. This will help to instil consumer confidence around receiving a high standard of safe and regulated service.
There have been calls for clear guidelines and safeguards to be put in place from within the industry and by consumers for many years and ministers have called for the introduction of a licensing scheme to be accelerated. Now, the intention is to consult with a broad range of industry stakeholders and members of the public to scope out a new licensing scheme which will be operated by local authorities.
The Consultation is seeking views on how the licensing scheme for non-surgical cosmetic procedures in England should be shaped, including:
Procedures to be captured under the regime will be classified by their potential complications and level of risk, which includes invasiveness and complexity.
These will be RAG-rated and classified as follows:
Procedures under the Amber RAG rating are currently set to be conducted either:
The main concerns for beauty practitioners are the perceived divide drawn between them and medical professionals, as well as the range of treatments that are regularly performed by experienced beauty practitioners. What is currently being proposed in the Consultation can be considered restrictive.
The parameters of these categories will need careful discussion and the views collected as part of the Consultation will help to shape that debate. Following the Consultation, we may see changes to which categories procedures fall into under the RAG ratings or requirements.
The current Consultation also proposes an age restriction for those who wish to undergo a cosmetic procedure. People under the age of 18 will not be able to undergo a procedure unless it’s approved by a GMC-registered doctor and carried out by a specified healthcare professional.
This age restriction would bring age requirements in-line with earlier legislation — namely the Botulinum Toxin and Cosmetic Fillers (Children) Act 2021 (BTCF Act) — as well as age restrictions for other procedures such as tattoos, teeth whitening and sunbed use.
The BTCF Act — which came into force in 2021 — made it an offence for botulinum toxin or the injection of fillers for cosmetic purposes to be administered to a person under 18 years of age in England.
In addition, the Advertising Standards Authority’s code on advertising practice (CAP) also provides guidance on marketing communications relating to surgical and non-surgical cosmetic procedures. The CAP guidance clearly sets out a prohibition on advertising prescription-only medicines (such as Botox) and that any ads involving the marketing of surgical and non-surgical cosmetic interventions must not be directed at those aged under 18. Anyone falling foul of the guidance could face sanctions.
The Consultation requires effective collaboration between beauty practitioners and the Government to ensure that it’s impactful and strikes a balance between agreed standards (which will allow the industry to thrive and innovate) and maintaining the confidence, trust and safety of consumers.
A further consultation is expected to be carried out at a later date to discuss the education standards for each type of licence, as well as licensing fees and insurance requirements (among other matters).
The industry’s voice will help to shape the licensing regime. This is now the final call to both industry professionals and consumers who have undergone procedures to submit their views to the Consultation.
Responses must be filed online by 11:59 on 28 October 2023.
Our lawyers are experienced in advising a range of beauty, fashion and healthcare clients.

We explore recent examples of how brands are responding to dupe culture and outline practical steps that retail businesses can take to protect their brand.
Read more

We’ve supported IMT Matcher in pursuing strategic partnership with ReproTech LLC, creating a global leader in IVF treatment technology.
Read more

We explore the legal and practical considerations of the nominated individual role, with a particular focus on dental practices.
Read more